Abstract
Background Acute graft-versus-host disease (aGvHD) is a major cause of early morbidity after allogeneic hematopoietic cell transplantation (alloHCT). Despite the use of prophylactic immunosuppressive therapy, aGvHD occurs in 40% -60% of matched related and unrelated alloHCT and severe cases contribute to non-relapse mortality.
RGI-2001 is a liposomal glycolipid that binds CD1d receptor of antigen-presenting cells (APC) resulting in activation of invariant natural killer (iNKT) cells. This interaction results in a cytokine-dependent Treg proliferation with subsequent modulation of the GvHD pathogenic cascade. RGI-2001 is being evaluated for the potential to reduce or prevent aGvHD after alloHCT. Earlier studies had shown that a single dose of RGI-2001 given on the day of hematopoietic cell transplantation was safe and potentially contributed to aGvHD prevention.
Methods: RGI-2001-003 (NCT04014790) is an open-label, multi-center phase 2b study to evaluate the potential efficacy and safety of RGI-2001 when added to calcineurin inhibitor with methotrexate or mycophenolate mofetil (without T-cell depletion) for the prevention of aGvHD in subjects following myeloablative alloHCT. RGI-2001 was administered in a 30-minute infusion at a dose of 100 ug/kg IV infusion weekly x 6 doses, starting on the day of transplant (Days 0, 7, 14, 21, 28, 35). The primary endpoint of the phase 2b study was grade II-IV acute GVHD by Day 100.
Results: A total of 53 subjects were screened and 49 treated at 7 U.S. transplant centers. Median age was 52 (range 21-65). The most common underlying indications for alloHCT were AML(n=27, 55%), ALL (n=11, 22%), and MDS (n=7, 14%). Donors were HLA-matched related (n=15), match unrelated (n=28) or mismatched unrelated (n=6, 5 HLA DQ and 1 HLA-A). Graft source was peripheral blood progenitor cells (n=40) or bone marrow (n=9). Common myeloablative conditioning regimens included Fludarabine/ Busulfan (81.6%), Cyclophosphamide/TBI (12.2%), and Cytarabine/Cyclophosphamide/TBI (4%). All subjects received tacrolimus/methotrexate for GvHD prophylaxis.
Preliminary results are reported herein with all 49 subjects having completed study day 100. There were no reported serious infusion reactions and one subject received 5 of 6 doses due to a treatment related AE (increased ALT, grade 3). Treatment-emergent AE (TEAE) related to RGI-2001 (> 5%) were diarrhea (12%), nausea (12%), abdominal pain (8%), increased ALT (6%), increased alkaline phosphatase (6%), and rash (6%). Grade 3/4 related TEAE (>1 subject) were decreased neutrophil count (6%), decreased platelet count (4%), anemia (4%), stomatitis (4%) and decreased appetite (4%). Serious adverse events reported were sepsis, upper respiratory tract infection, diarrhea, headache, VOD, pelvic pain and DVT; none were deemed related to RGI-2001. There were no cases of engraftment failure.
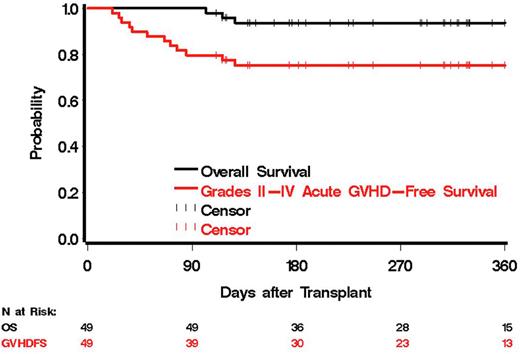
All 49 patients had at least 100 days of follow-up (FU), and 39 of 49 had complete FU to day 180. The median FU of survivors was 310 days (range, 111-365). Through day 100, there were 10 cases of grades II-IV aGvHD [20.4% (95% confidence interval 10.2-34.3%)], two of which were grades III-IV aGvHD [(4.1% (0.5-14.0%)]. One subject died from aGvHD due to GI bleed. There have been 3 relapses, 3 deaths, and 7 cases of moderate (0 severe cases) chronic GVHD (cGVHD) by last contact. Day-180 estimates of overall survival (OS), grades II-IV aGvHD-free survival (GFS), and relapse were 93.6% (81.6-97.9%), 75.2% (60.5-85.1%), and 4.1% (0.7-12.6%), respectively. Figure 1 summarizes the probability of OS and GFS as a function of time.
Conclusions: Intravenous administration of RGI-2001 added to standard-of-care tacrolimus / methotrexate showed promising efficacy in the prevention of clinically significant aGVHD with a favorable safety profile. A phase 3 study is planned.
Disclosures
Chen:Actimium: Consultancy; Celularity: Consultancy; Equillium: Consultancy; Gamida Cell: Consultancy; Incyte: Consultancy; Jasper: Consultancy; Novartis: Consultancy. Saad:Kite/Gilead: Consultancy; Magenta Therapeutics: Consultancy; Incyte: Consultancy; CareDx: Consultancy; In8Bio Inc: Patents & Royalties. Schiller:Regimmune: Research Funding; Forma: Research Funding; Deltafly: Research Funding; Celgene: Consultancy, Research Funding, Speakers Bureau; AstraZeneca: Honoraria; Mateon: Research Funding; Incyte: Other: speaker fees, Research Funding, Speakers Bureau; Constellation: Research Funding; Arog: Research Funding; Gilead: Research Funding; Millennium: Research Funding; Onconova: Research Funding; Johnson & Johnson: Current equity holder in publicly-traded company; Cellerant: Research Funding; Genentech-Roche: Research Funding; Geron: Research Funding; Novartis: Honoraria, Other: Speaker fees, Research Funding; Samus: Research Funding; Gamida: Research Funding; Jazz: Consultancy; Deciphera: Research Funding; Trovagen: Research Funding; Pfizer: Research Funding; Cellectis: Research Funding; Karyopharm: Research Funding, Speakers Bureau; Amgen: Current equity holder in publicly-traded company, Honoraria; PreCOG LLC: Research Funding; AVM Biopharma: Research Funding; Glycomimetics: Research Funding; Agios: Consultancy, Honoraria; Sellas: Research Funding; Ono Pharma: Honoraria; FujiFilm: Research Funding; Stemline: Research Funding; Kite, a Gilead Company: Research Funding, Speakers Bureau; AltruBio: Research Funding; Actinium: Research Funding; Cyclacel: Research Funding; Actuate: Research Funding; Janssen: Research Funding; Stemline: Speakers Bureau; Daiichi-Sankyo: Research Funding; Medimmune: Research Funding; CTI: Research Funding; Astellas: Research Funding, Speakers Bureau; Bristol Myers Squibb: Current equity holder in publicly-traded company, Speakers Bureau; AbbVie: Research Funding, Speakers Bureau; Sangamo: Research Funding; Takeda: Research Funding; Tolero: Research Funding. Yared:Kite: Honoraria. Assal:Incyte Corporation: Membership on an entity's Board of Directors or advisory committees, Research Funding; Guidepoint Inc: Consultancy; Alphasights: Consultancy; Curio Science: Consultancy. Lane:Regimmune Corporation: Consultancy. Defilipp:Taiho Oncology: Research Funding; Syndax Pharmaceuticals: Consultancy; Kadmon: Consultancy; Omeros: Consultancy; Regimmune: Research Funding; Incyte: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal